IMMUNISATION OP-ED
Vaccine safety surveillance in South Africa — how the system works in the individual and public health interest
Vaccines are among the 20th century’s most successful and cost-effective public health tools for preventing disease, hospitalisation and death. Timely immunisation is one of the most important ways for people to protect themselves and those around them from serious diseases such as Covid-19, measles, pneumonia, tetanus and pertussis. The World Health Organization (WHO) estimates that immunisation prevents two to three million deaths every year.
Immunisations are for people of all ages throughout their life course — from pregnancy to newborns, through infancy, for toddlers, adolescents, adults and the elderly.
Below, we explain some of the principles, procedures and ethics governing vaccine development, registration, use and monitoring for safety.

Shoppers and motorcyclists pass a stalls at Kebayoran Lama market in Jakarta, Indonesia, on Thursday, July 15, 2021. Photographer: Dimas Ardian/Bloomberg
Passive versus active immunity from infection and/or disease
Immunity can be acquired through active or passive immunity.
Active immunity is when a non-immune person responds to vaccination or natural infection by mounting an immune response. The infection is cleared and upon subsequent exposure, a response is mounted based on memory and infection is averted. Active immunity thus results in long-lasting protection.
Some people believe that naturally acquired immunity is better than the immunity provided by vaccines. However, natural infections can cause serious complications and be fatal. Again, this is exactly why vaccines are used, to prevent the long-term negative effects of diseases, of which there is ample evidence.
Passive immunity results when a non-immune person receives immunoglobulins from an immune individual, such as during pregnancy, when maternal antibodies are transferred to the foetus via the placenta, or to the infant via breast milk. As a result, infection is prevented if the infant is exposed to natural infection while maternal antibodies are still circulating.
However, upon subsequent exposure when maternal antibodies are no longer in circulation, the child is non-immune and will not avert infection. Passive immunity, therefore, provides only temporary protection.
This type of immunity is important, which is why pregnant women are vaccinated, so as to protect their babies until they are old enough to get vaccinated.
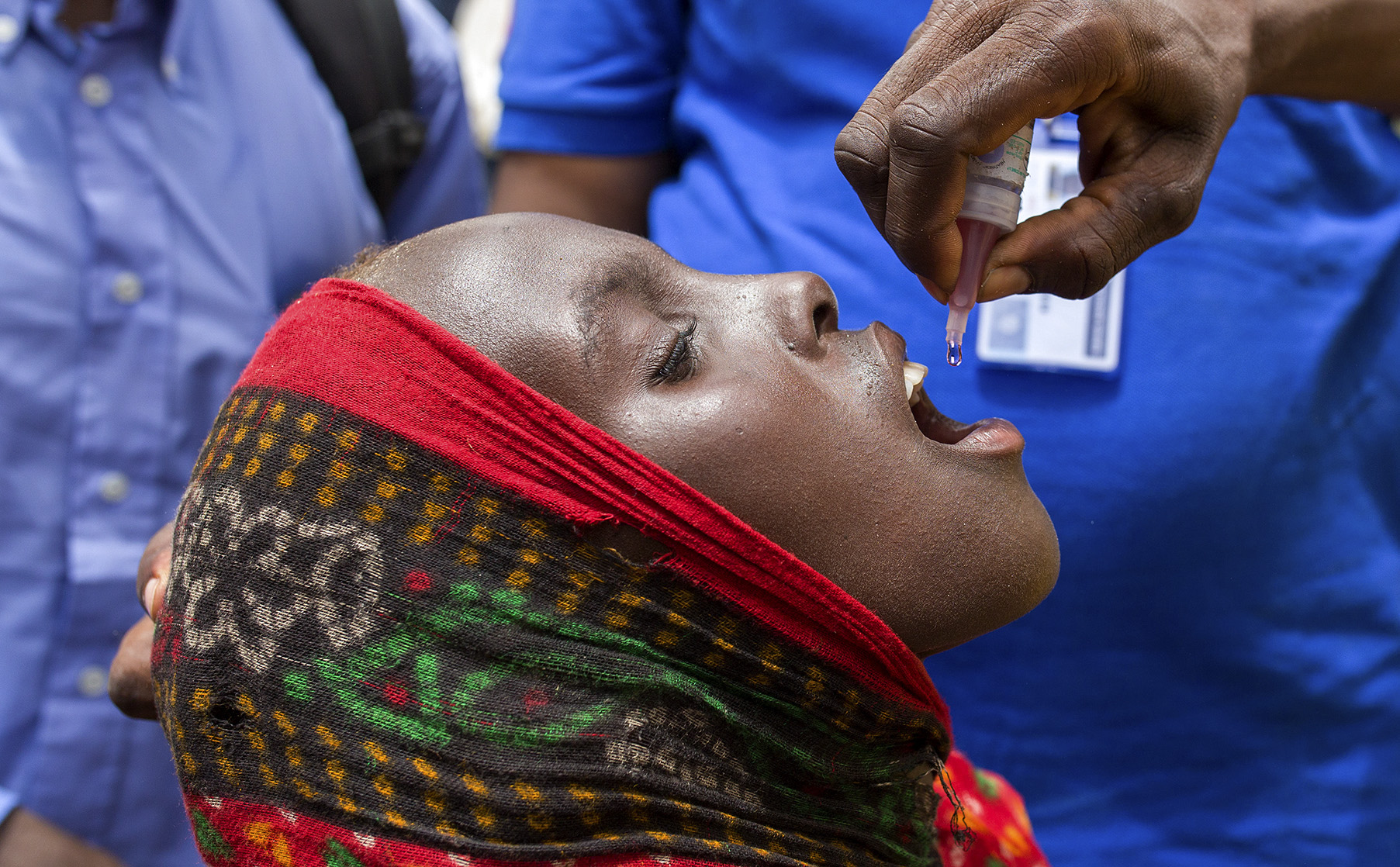
A girl receives two drops of oral polio vaccine during market day on the island of Ngorerom on Lake Chad. Lake Chad, 2019. (Photo: © Christine McNab/UN Foundation)
Acquiring immunity through vaccination
The basic principle of vaccination is to activate the body’s defences by mimicking the invasion of a specific infectious agent, which will trigger the person’s defence mechanisms just like a real infection.
Vaccines cannot cause disease because they are either a form of the micro-organism that has been weakened (attenuated) in some way so that it no longer causes clinical symptoms; or a killed or inactivated whole micro-organism, or just a part of the micro-organism. Either way, the infectious agent has lost its ability to cause disease, but it can still stimulate a strong immune response, providing protection against future exposure to the real infectious agent.
Once vaccinated, the body will mount a specific primary immune response with the establishment of immunological memory (in most cases). When coming into contact with the real infectious agent, the body mounts a rapid and potent secondary response that will control and eliminate the infectious agent before it has had time to establish itself and provoke clinical disease. Vaccination thus prevents the disease if exposure to the organism occurs at a later stage.
The ideal vaccine is one which is
- Highly immunogenic (inducing a rapid, strong and specific immune response);
- Totally effective in a single dose;
- Safe to administer, having no adverse reactions or side effects;
- Capable of inducing life-long immunity in 100% of the population;
- Stable under non-stringent storage conditions;
- Genetically stable (specifically referring to live vaccines);
- Easily administered; and
- Affordable to the intended population.
In reality though, no vaccine is “ideal”. Most vaccines are highly immunogenic, but no vaccine is 100% safe and 100% effective. For most vaccines, lifelong protection usually requires booster doses.
(Photo: theconversation.com / Wikipedia)
Common, rare and very rare adverse reactions
Like all medicines, vaccines, including the Covid-19 vaccines, may cause side effects, also known as adverse reactions. It is normal and expected to experience side effects after being vaccinated, as it is part of the body’s immune response, showing that it is building protection against the pathogen.
This reaction is not the same for all vaccinees, as people’s immune systems react in different ways, which is why some people experience no side effects at all, while others do experience one or more side effects with intensity varying as well. Individuals may therefore respond differently to the same vaccine or medicine, making it difficult to predict whether an individual will experience any adverse reaction.
A vaccine is only authorised by regulatory authorities for use in the general population when the vaccine has a good benefit-risk profile. This means that the benefits of the vaccine in preventing severe disease, hospitalisation and death from an infectious disease, far outweigh these expected side effects. Vaccines that are shown to have serious side effects during clinical trials will not be authorised by the regulatory authority.
Very common or frequent side effects or adverse reactions are usually minor and are expected, as they are known from clinical trials in which vaccines are tested on tens of thousands of people. Examples include redness, pain at the injection site, slight fever, dizziness/fatigue and nausea. Very common side effects like headaches are expected in at least one in every 10 people and common side effects like nausea in at least one in every 100 people. These non-serious, mild side effects are short-lived, meaning they occur within approximately six hours of vaccination, could peak at around 24 hours and usually resolve within 2-3 days. In most cases, there is no need to treat mild side effects.
Examples of severe adverse reactions include severe diarrhoea and vomiting, putting the person at risk of dehydration, especially small children and the elderly, very high fever, severe swelling or an abscess at the injection site, or an allergic reaction such as swelling of the lips. Although these reactions are severe, they are non-serious and can be managed successfully with medical treatment.
When any of the minor reactions become severe and/or last for more than 2-3 days, the vaccinee must seek medical assistance at the nearest clinic, hospital or general practitioner. The same applies to any side effect or adverse event that is of concern to the vaccinee.
Rare and very rare adverse reactions may occur after vaccination. These adverse reactions are uncommon, not expected and can only be detected when large populations are vaccinated and if adverse events are being reported.
Rare events could be mild, but they could also be severe and serious, requiring medical attention or admission to hospital. Rare events will appear in less than one in 10,000 people and extremely rare events will appear among 1-10 in a million vaccinated people. Examples include anaphylaxis, which is a very severe allergic reaction, usually occurring within a few minutes of vaccination. Anaphylaxis can be managed safely, which is why vaccinees must be observed for 15 minutes after vaccination.
Other examples of rare events include a rare form of blood clots, mild heart inflammation, and Guillain Barré syndrome. These rare events are often more common in certain age groups or populations and can also be managed successfully if they are identified early, which is why people should immediately report to a healthcare facility should they experience anything of concern.
What is important about seeking medical care, is that several people experience illnesses after being vaccinated, that is unrelated to the vaccination. During the Covid-19 pandemic, hundreds of thousands of people were vaccinated daily, including elderly people and those with comorbidities, hence other illnesses unrelated to the vaccine are expected. Furthermore, vaccinated people may be incubating another infectious disease at the time of vaccination, e.g., Covid-19, pneumonia or malaria.

Residents line up to receive Covid-19 vaccines at Naledi Vaccination Site on November 11, 2021 in Soweto, South Africa. Photo by Gallo Images/Papi Morake)
Monitoring the safety of vaccines for signal detection
When a vaccine is rolled out to millions or billions of people, it is important to look for safety signals, but it is also important to determine whether the signal identified is caused by or linked to the vaccine, or whether it is just because other illnesses happen when you roll out vaccines to millions of people.
A large pool of reports is necessary to identify rare and very rare vaccine adverse reactions and signals.
During the Covid-19 pandemic, the South African Health Products Regulatory Authority (Sahpra) launched the Med Safety App to enable electronic reporting of adverse events following immunisation (AEFI), to strengthen the safety surveillance system in the country. An advantage of electronic reporting through the Med Safety App is that South Africa’s data on reported adverse events are pooled with the data from other African countries (initially Nigeria, Ghana and Ethiopia; recently also Kenya), through the African Union Smart Safety Surveillance (AU-3S) programme.
Signal detection runs are conducted on the data every two weeks, with support from the medicines regulatory authority in the UK, followed by a group of experts representing the countries reviewing the signal detection reports. Throughout the Covid-19 pandemic to date, no new safety signals have been identified for any of the Covid-19 vaccines used in the initial four African countries, other than the adverse reactions already known and documented in the vaccines’ product information.
The outcomes of signal detection and causality assessments are continuously reviewed by regulatory authorities in Africa and across the globe. When a positive causal association between an adverse event and a vaccine is confirmed, the new adverse event is then classified as an adverse reaction or a side effect. Thereafter the benefit-risk ratio is calculated, and if this remains positive because it was not a known side effect or adverse reaction included in the product information, changes to the product information will be made, which will include the expected risk of such an adverse reaction.
South Africans can rest assured that Sahpra closely monitors the performance of all vaccines that have been authorised and is highly responsive to any safety signals. Should a signal be identified, they will take appropriate action such as amendment of the product information or restricting access to the vaccine for certain population groups.
For example, in 2021, Sahpra immediately paused the roll-out of the Janssen Covid-19 vaccine, because a safety concern about a rare blood-clotting event affecting six US women, representing 0.00008% of the vaccinated population, was flagged. As a result, the product information of the vaccine was amended to include a warning about this risk.
The safety of vaccines is continuously monitored, which is why people are encouraged to report all adverse events that are of concern to them, through the correct reporting channels.
Importance of reporting adverse events following immunisation
When a vaccine comes on the market, the side effects or adverse reactions identified during the clinical trials are listed in the product information of the vaccine. These side effects are thus known to be caused by the vaccine but still need to be monitored when the larger population is vaccinated, to assess that the rates of the side effects identified in the clinical trials remain low and acceptable. Some rare or unknown adverse reactions may not be seen until large numbers of the population have been vaccinated.
On the other hand, an AEFI is any untoward or unpleasant medical event that happens after a person has received a vaccine. There are various reasons why an adverse event may occur; however, these adverse events may or may not have been caused by the vaccine. These events must be reported and investigated to determine if they are related to vaccination, or if they happened coincidentally after vaccination, and would have happened if the person was not vaccinated.
There are also adverse events of special interest, which are pre-specified medical events that have the potential to be linked with a vaccine product (eg, anaphylaxis), serious events related to vaccine immunogenicity or events that are potentially specific to special populations. These events are short-listed by global authorities such as the World Health Organization and must be carefully monitored.
Reporting AEFIs helps to identify any unknown or poorly described adverse events, as causality assessment is used to determine whether there is a causal relationship between the adverse event and the vaccine, whether the adverse event was caused by any of these other reasons mentioned or whether it might be a signal of a possible new adverse reaction that must be investigated further or monitored in future. While some mild and short-lasting side effects are acceptable, severe and serious adverse reactions are not acceptable and should be fully investigated to understand if the vaccination was responsible for the event.
The difficulty with monitoring potential vaccine side effects is that detecting them is entirely dependent on people recognising the symptoms and reporting all adverse events through the correct reporting channels.
The benefits of reporting and monitoring adverse events are to ensure the safety of the vaccines, hence preventing harm to other people who may experience similar reactions to a specific vaccine or medicine. It also ensures an accurate safety profile of a specific vaccine or medicine.
For example, should a previously unknown adverse reaction caused by the vaccine be identified, Sahpra could request that the product information be changed, to reflect the particular adverse reaction and include specific contraindications for its use, special precautionary measures that should be taken or special warnings with the use of the vaccine or the medicine.
In October, the World Health Organization awarded the South African Health Products Regulatory Authority’s vaccine reviewing system a level three maturity. This means that all the processes needed to ensure that people will get safe immunisations are in place. (Photo: Sigrid Kite)
Practical steps: How and where to report an adverse event following immunisation
The safety of vaccines is continuously monitored, which is why people are encouraged to report all adverse events that are of concern to them. All members of the public and healthcare workers may report AEFI using the Med Safety App. Any adverse events can also be reported directly to any healthcare facility.
When reporting an AEFI, submission of all the necessary information about the AEFI is extremely important because a causality assessment can only be conducted if sufficient information is available.
If a case is reported without any information to trace it, for example, without an ID number or contact details, no investigation and subsequent causality assessment can be conducted.
The same applies when a death has occurred after vaccination and the death certificate states “natural causes”. If there was no postmortem examination before the burial, or the information provided is incomplete, e.g., no clinical history available, then it will be impossible to determine any causality. Hence it is of utmost importance that when a case is reported, all information requested must be provided.
Investigation and causality assessment of serious adverse events following immunisation
A common misperception, even among highly educated people, is that an “event following immunisation” was “caused by immunisation”. This misperception is often fuelled by the spread of misinformation about vaccines, especially on social media. People should guard against trusting this kind of misinformation more than trusting scientific evidence.
A challenge in interpreting AEFIs is that serious disease events such as stroke, heart attack and blood clots occur in the general population, particularly among older persons. When they occur after a Covid-19 vaccination, it does not mean that the vaccine was the cause of the event. A full investigation and causality assessment is essential to understand the connection between vaccination and adverse event.
For this reason, all severe and serious adverse events that are reported are fully investigated by a multidisciplinary team of provincial health officials by obtaining details of vaccination, medical records and laboratory investigations following the adverse event. These investigations are essential and must be done thoroughly based on all the information that is required because causality assessment can only be conducted if all the required information about a case is available.
Once investigations are completed, the case investigation form with all supporting documentation is submitted to the National Department of Health (NDoH) office, where they verify that all required documentation is available and if so, submit the case to the National Immunisation Safety Expert Committee (Nisec).
Nisec is an independent committee appointed by the minister of health to conduct causality assessment, by evaluating reports and to determine in each case whether the vaccine is responsible for the reported adverse event, or whether it occurred by chance, coincidentally following the time of vaccination. The committee consists of medical experts including pharmacists, pharmacovigilance experts, infectious disease specialists, paediatricians, EPI programme experts, immunologists, allergologists, microbiologists, neurologists, pathologists and public health specialists.
Experts review the AEFI reports and evaluate the events using the WHO global vaccine safety tools and step-wise methodology, and categorise the event as one of the following:
- Consistent with a causal association to immunisation, which could be due to the vaccine product, vaccine quality or defects, errors in administering the vaccine, or related to anxiety or stress around the process of vaccination;
- Indeterminate, meaning temporally associated with vaccination but without sufficient definitive evidence for the vaccine causing the event or if available evidence indicates conflicting trends for and against the vaccine causing the event;
- Coincidental event (due to another underlying condition or unrelated health event); or
- Unclassifiable in the case of inadequate information being available.
Nisec reports the causality assessment findings to the minister of health through the NDoH, who in turn reports to the provincial departments of health and to Sahpra. The NDoH and Sahpra do not have any influence on the causality assessment process apart from providing secretarial support to Nisec, which is important for governance.
Schoolchildren receive an injection of the Measles and Rubella vaccine during a vaccination program drive for school children in Banda Aceh, Indonesia, 28 July 2022. EPA-EFE/HOTLI SIMANJUNTAK
No-Fault Compensation Scheme
Should an event be classified by Nisec as consistent with a causal association to the Covid-19 immunisation, the case is referred to the Covid-19 Vaccine Injury No-Fault Compensation (NFC) Scheme as published in April 2021 by the Cogta minister, Dr Nkosazana Dlamini Zuma. Note that these regulations will remain in effect until the last dose of Covid-19 vaccine has been used.
The NFC Scheme has been established to:
- Provide access to compensation for eligible persons who suffered a severe vaccine injury (harm, loss or damage) due to the administration of an applicable vaccine at an official vaccination site; and
- Provide access to the dependant of a deceased person, who has suffered harm, loss or damage caused by the death of the deceased person, whose death was caused by a vaccine injury due to the administration of an applicable vaccine at an official vaccination site.
Claims are assessed by a panel of experts, forming the Adjudication Panel.
By 15 April 2023, the NFC had finalised 42 claims and made payment for one vaccine-related death and one temporary disability. One other death claim and one temporary disability claim have been approved but not yet paid. Three more have been adjudicated and await authorisation by the director-general to make payments. Altogether, 42 cases are either paid or in the pipeline from Nisec awaiting claim adjudication or payment authorisation by the director-general.
In the event that a claimant is not satisfied with the outcome, the claim will be assessed by the Appeals Panel, which has different panel members than the Adjudication Panel.
In the event that a payment is approved, either by the Adjudication Panel or the Appeals Panel, then the sum to be paid is calculated using a quantum according to the methodology outlined in Schedule 6 (Compensation Table) of the regulations for the Covid-19 Vaccine Injury No-Fault Compensation Scheme.
The NDoH and Nisec do not have any influence on the outcome of the claims assessment process. A scheme administrator has been appointed to provide secretarial support to the two panels and to provide administrative support in finalising claims, as well as communication with claimants and panel members.
The roll-out of the Covid-19 vaccines in response to the Covid-19 pandemic required immediate strengthening of vaccine safety surveillance in South Africa. This was successfully done by Sahpra through the introduction of the Med Safety App, allowing spontaneous electronic reporting of AEFIs by healthcare workers and the public. Rates of serious adverse events remain very low for the Covid-19 vaccines used in the country. Furthermore, because South Africa is part of the AU-3S programme, it provides assurance of the safety of the vaccines based on data collected from the African continent.
It remains important to balance the very small risk of serious adverse events against the much larger risks of severe disease, hospitalisation and death from Covid-19. The small number of people who have suffered serious adverse events from Covid-19 vaccination must be seen in the context of the millions of lives saved through Covid-19 vaccination and the number of excess deaths which are attributable either directly or indirectly to Covid-19 disease.
Lastly, detailed reporting of any AEFI, especially serious events, is essential, as this allows analysis and causality assessment of these events, assisting in understanding them better and monitor vaccine safety. DM/MC
Prof Hannelie Meyer is the chair of the National Immunisation Safety Expert Committee; Marione Schönfeldt, Prof Nicholas Crisp and Magda Fourie are all with the National Department of Health.




















 Become an Insider
Become an Insider
Yes, the Covid 19 vaccines were very important in 2021 to prevent serious illness but now they should not be given to all and sundry. There is natural immunity against Covid and the Covid strain we have at the moment is mild, whereas there is more and more information coming out about the vaccines.
Who makes up the panel that adjudicates on claims? And why is the pharmaceutical companies not held liable, but the South African government must pay?