How do atoms form? – Joshua (7), Shoreview, Minnesota, US
Richard Feynman, a famous theoretical physicist who won the Nobel Prize, said that if he could pass on only one piece of scientific information to future generations, it would be that all things are made of atoms.
Understanding how atoms form is a fundamental and important question, since they make up everything with mass.
The question of where atoms come from requires a lot of physics to be answered completely – and even then, physicists like me only have good guesses to explain how some atoms are formed.
What is an atom?
An atom consists of a heavy centre, called the nucleus, made of particles called protons and neutrons. An atom has lighter particles called electrons that you can think of as orbiting the nucleus.
The electrons each carry one unit of negative charge, the protons each carry one unit of positive charge, and the neutrons have no charge. An atom has the same number of protons as electrons, so it is neutral − it has no overall charge.
Now, most of the atoms in the universe are the two simplest kinds: hydrogen, which has one proton, zero neutrons and one electron; and helium, which has two protons, two neutrons and two electrons. Of course, on Earth there are lots of atoms besides these that are just as common, such as carbon and oxygen, but I’ll talk about those soon.
An element is what scientists call a group of atoms that are all the same, because they all have the same number of protons.
The first atoms form
Most of the universe’s hydrogen and helium atoms formed about 400,000 years after the Big Bang, which is the name for when scientists think the universe began, about 14 billion years ago.
Why did they form at that time? Astronomers know from observing distant exploding stars that the size of the universe has been getting bigger since the Big Bang. When the hydrogen and helium atoms first formed, the universe was about 1,000 times smaller than it is now.
/file/dailymaverick/wp-content/uploads/2025/07/p26-Atoms-inset.jpg)
Before this time, the electrons had too much energy to settle into orbits around the hydrogen and helium nuclei. So, the hydrogen and helium atoms could form only once the universe cooled down to something like 2,760 degrees Celsius. For historical reasons, this process is misleadingly called recombination, but combination would be more descriptive.
The helium and deuterium − a heavier form of hydrogen − nuclei formed even earlier, just a few minutes after the Big Bang, when the temperature was above 556 million degrees Celsius. Protons and neutrons can collide and form nuclei like these only at very high temperatures.
Scientists believe that almost all the ordinary matter in the universe is made of about 90% hydrogen atoms and 8% helium atoms.
How do more massive atoms form?
So, the hydrogen and helium atoms formed during recombination, when the cooler temperature allowed electrons to fall into orbits. But you, I and almost everything on Earth is made of many more massive atoms than just hydrogen and helium. How were these atoms made?
The surprising answer is that more massive atoms are made in stars. To make atoms with several protons and neutrons stuck together in the nucleus requires the type of high-energy collisions that occur in very hot places. The energy needed to form a heavier nucleus needs to be large enough to overcome the repulsive electric force that positive charges, like two protons, feel.
Protons and neutrons also have another property – kind of like a different type of charge – that is strong enough to bind them together once they are able to get very close together. This property is called the strong force, and the process that sticks these particles together is called fusion.
Scientists believe that most of the elements from carbon up to iron are fused in stars heavier than our sun, where the temperature can exceed 556 million degrees Celsius – the same temperature that the universe was when it was a few minutes old.
But even in hot stars, elements heavier than iron and nickel won’t form. These require extra energy, because the heavier elements can more easily break into pieces.
In a dramatic event called a supernova, the inner core of a heavy star suddenly collapses after it runs out of fuel to burn. During the powerful explosion this collapse triggers, elements that are heavier than iron can form and get ejected into the universe.
Astronomers are still figuring out the details of other fantastic stellar events that form larger atoms. For example, colliding neutron stars can release enormous amounts of energy – and elements such as gold – on their way to forming black holes.
Understanding how atoms are made requires learning a little general relativity, plus some nuclear, particle and atomic physics. But to complicate matters, there is other stuff in the universe that doesn’t appear to be made from normal atoms at all, called dark matter. Scientists are investigating what dark matter is and how it forms. DM
First published by The Conversation.
Stephen L Levy is associate professor of physics, applied physics and astronomy at the State University of New York at Binghamton.
This story first appeared in our weekly Daily Maverick 168 newspaper, which is available countrywide for R35.





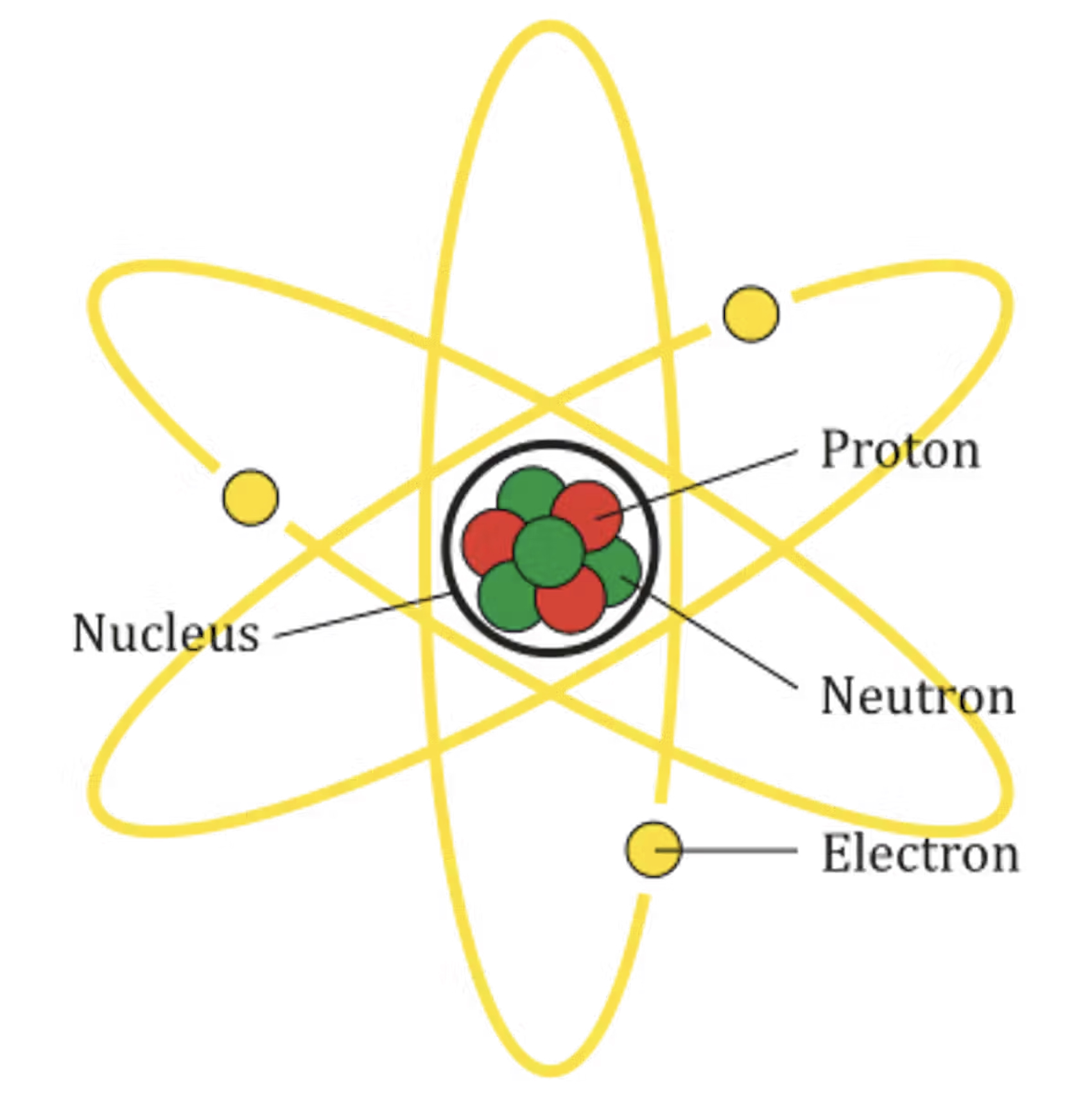 An atom consists of positively charged protons, neutrally charged neutrons and negatively charged
electrons. (Image: AG Caesar / Wikimedia Commons)
An atom consists of positively charged protons, neutrally charged neutrons and negatively charged
electrons. (Image: AG Caesar / Wikimedia Commons)