SPOTLIGHT IN-DEPTH
South Africa’s remarkable TB clinical trial capacity — here’s why so much research can be done here
Several of the world’s most important tuberculosis clinical trials of the past two decades were done in part or entirely in South Africa. Tiyese Jeranji chatted to some leading researchers about where the country’s clinical trial capacity comes from and what is needed to maintain and improve it.
These trials include several that helped establish the safety and efficacy of the medicines the World Health Organization (WHO) now recommends for the treatment of drug-resistant forms of TB.
It also includes several trials of experimental TB vaccines, one of which was a landmark phase 2 trial of the M72 vaccine – it seems likely the phase 3 trial will also include study sites in South Africa.
According to Global Data (a research and consulting company), of the 1,925 clinical trials started and completed in sub-Saharan Africa from 2012 to 2023 (not just TB trials), 930 were in South Africa. Roughly half of the clinical trials in the region related to infectious diseases. Africa as a whole had just more than 5,000 clinical trials in the period – 2.2% of the global tally. With more than 2,900 clinical trials, Egypt accounted for almost six out of every 10 conducted on the continent.
One reason so much TB and HIV research can be done in South Africa is quite simply that we have large epidemics of both diseases. About 13% of the population is living with HIV and the country is on the WHO’s lists of 30 high TB burden and 30 high DR-TB burden countries. The WHO estimates that about 280,000 people in South Africa fell ill with TB in 2022 and 54,000 died of the disease.
Yet, while some TB clinical trials are conducted in other countries with large TB epidemics, South Africa seems to be something of an outlier in terms of just how many TB clinical trials are conducted here. Clearly other factors involved.

Dr Francesca Conradie, an infectious diseases researcher in the School of Clinical Medicine at Wits, who led the landmark NiX trial. (Photo: Supplied / Spotlight)
The research environment
Dr Angelique Luabeya, chief research officer at the South African Tuberculosis Vaccine Initiative, says the country’s capacity to run successful clinical trials is derived from a combination of factors such as infrastructure, skilled human resources, funding, regulatory and ethical oversight, community engagement and sustained political and organisational commitment.
Similar factors are pointed out by Dr Francesca Conradie, an infectious diseases researcher in the School of Clinical Medicine at Wits. She says South Africa has a high burden of TB disease, an excellent laboratory service and a robust regulatory environment.
“We can make the diagnosis of TB and trials can be run to the highest standards. Our TB programme is excellent and engaged in the research agenda,” Conradie says.
Conradie led the landmark NiX trial that upended traditional notions of TB treatment by showing that tough-to-treat forms of TB could successfully be treated with just three drugs taken for six months – previously four or more drugs were used for 18 to 24 months, and cure rates were much lower.
The ability to carry out high-quality clinical studies is affected by ‘the wider South Africa macrocosm’ becoming unstable because of things such as electricity interruptions, skills shortages, immigration and concerns about corruption.
In South Africa, clinical trials are regulated by the South African Health Products Regulatory Authority (Sahpra). TB clinical trials in the country are typically funded by entities such as the US National Institutes of Health, the European and Developing Countries Clinical Trials Partnership, the Bill and Melinda Gates Foundation, and in some limited cases by the South African government through the South African Medical Research Council or the Department of Science and Innovation (this Treatment Action Group report shows who funds TB research around the world).
Dr Limakatso Lebina, clinical trials unit lead at the African Health Research Institute (AHRI), stresses that South African researchers have extensive experience in conducting clinical trials and that in her view there are adequate regulations and sufficient infrastructure in place in the country.

Clinical trial units are research sites designed to evaluate new medicinal products and diagnostic tools, among other things. (Photo: Joyrene Kramer / Spotlight)
“As part of the global research world,” Lebina says, “people conduct research across many countries and include diverse groups of populations to gather adequate evidence to change guidelines.”
Her last point hints at an important benefit of conducting TB trials in South Africa: It helps us understand how well experimental TB treatments work in our population. For example, given the country’s very high rates of HIV/TB co-infection, it is important for us that new TB treatments are tested in people with such co-infection rather than just in people who have TB, but who are not living with HIV.
The infrastructure
Laboratories, roads and electricity are all part of the infrastructure needed to make it all come together, as are so-called clinical trial units. As Lebina explains, these units are research sites designed to evaluate new medicinal products, diagnostic tools, surgical procedures, vaccines, nutritional measures and psychological interventions. They design, conduct and analyse studies ranging from phase one (that evaluate safety and pharmacodynamics in humans) to phase four (that continue to monitor side-effects after treatment has been approved).
Lebina says setting up a clinical trial unit requires specialised infrastructure and skilled personnel, depending on the disease focus and the type of studies that are planned. On the one hand there are several similarities between such units and a normal healthcare facility, “such as equipment to conduct medical examinations and management of medical emergencies and a pharmacy”. On the other, these units also require “specialist equipment such as freezers for storage of vaccines, easy access to a laboratory for immediate processing of specimens and regular calibration of equipment”.
Read more in Daily Maverick: What the new WHO TB numbers mean for South Africa
Factors such as closeness to laboratories and willingness of people in the area to participate in trials are considered when deciding on the location of clinical trial units. For this reason they tend to be in cities or large towns.
Lebina says AHRI is aiming to turn this around with a clinical trial unit launched in 2022 in Somkhele, in rural northern KwaZulu-Natal. The uniqueness of the unit “is that it is in a rural setting and gives access to participation in clinical trials to a population that is often neglected or overlooked and underrepresented in the conduct of clinical trials”.
“We believe inclusion of diverse groups of people in research is essential to develop safe and effective medications and to offer clinical trials as a treatment option for all patients in need, irrespective of where they live or their socioeconomic status,” Lebina says.
The unit has a staff of 50 medical doctors, nurses, pharmacists, clinical research assistants, data managers, laboratory personnel and administrative staff. Studies being conducted or planned at the unit include research on two new TB vaccines, HIV pre-exposure prophylaxis trials that combine innovative treatment delivery options, HIV treatment with newer regimens, and asthma treatment studies.
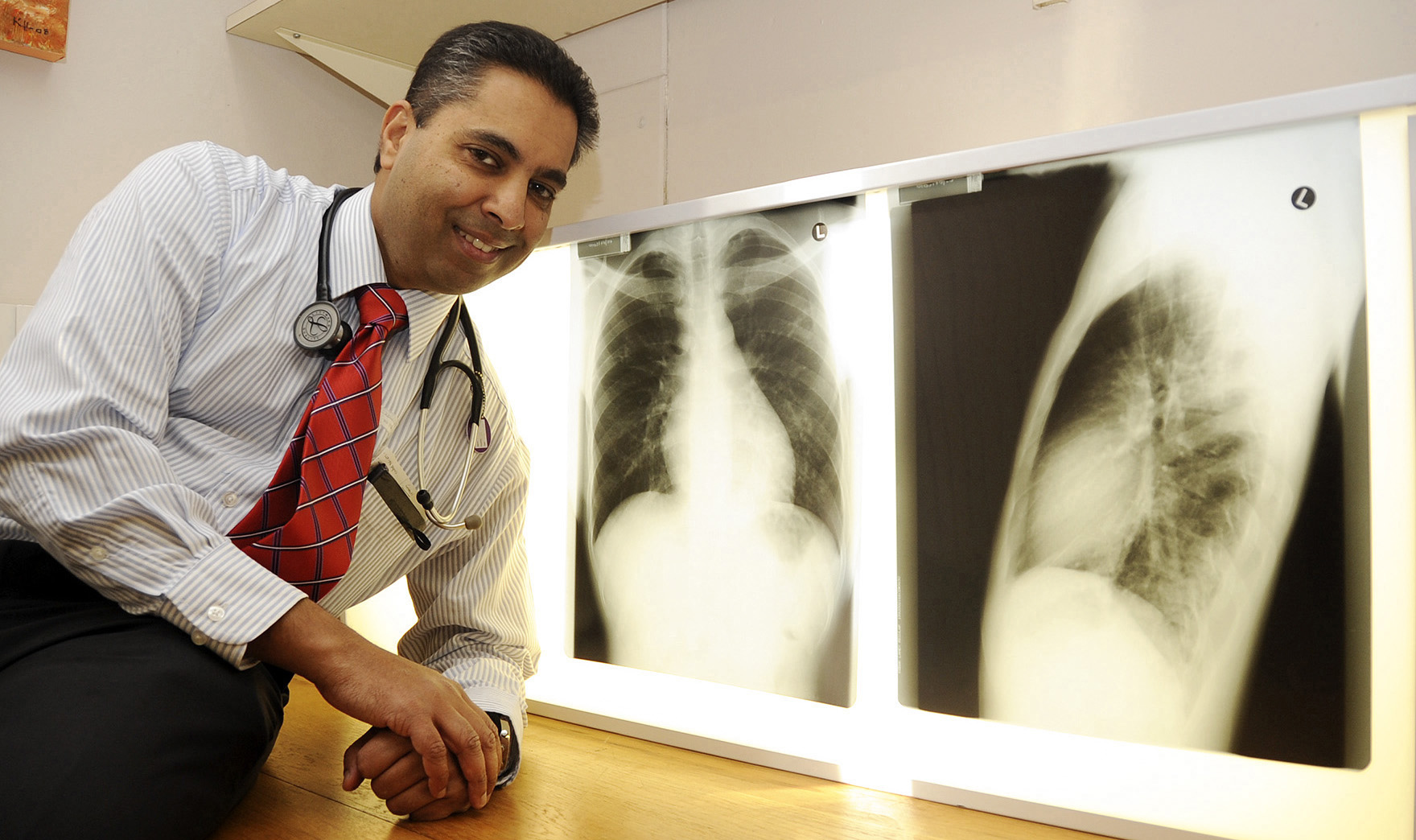
Professor and pulmonologist Keertan Dheda at Groote Schuur Hospital. (Photo: Supplied / Spotlight)
‘Problems with the system’
Professor Keertan Dheda, a pulmonologist and critical care specialist who heads up the division of pulmonology at Groote Schuur Hospital and the University of Cape Town, says that although South Africa has done well with clinical trials, there are major challenges on the horizon and problems within the system.
Chief among these is how long it takes to obtain the various sequential approvals required for these studies. Beyond ethical and Sahpra approval, there are often biosafety and provincial-level approvals, city-wide approvals to access healthcare facilities as well as export permits to ship samples. Huge delays can occur when obtaining some of these approvals, so a more streamlined process is needed, he said.
While challenges exist, the country has a resilient and growing research ecosystem, and there’s a concerted effort to strengthen its capacity further.
Dheda points out that crime has also affected research activities due to hijackings, research staff being robbed of reimbursement money for participants, and theft of computers from research staff. “This has escalated alarmingly in the past three years and is a major problem. This is especially true for trials involving TB and HIV because these often recruit participants in previously disadvantaged areas where crime is very high.”
The ability to carry out high-quality clinical studies is affected by “the wider South Africa macrocosm” becoming unstable because of things such as electricity interruptions, skills shortages, immigration and concerns about corruption.
Lebina says that setting up clinical trials comes with several challenges including delays in regulatory and administrative approvals, having very few skilled personnel in the trials, and limited funding.
Prospects for young researchers
Luabeya says South Africa offers ample opportunities for careers in the research fields, owing to the country’s strong legacy in medical research, especially in areas like HIV, TB and, more recently, Covid-19. It also has several renowned universities, research institutes and organisations dedicated to health research.
However, like many countries, South Africa does face challenges including funding constraints, administrative hurdles and “brain drain” where skilled professionals seek opportunities abroad, she adds.
“South Africa trains young researchers and offers them career paths in clinical trials. While challenges exist, the country has a resilient and growing research ecosystem, and there’s a concerted effort to strengthen its capacity further.”
According to Conradie, while the country is making progress in this area, there is still some way to go. “Being a clinical triallist is not yet seen as a career path. Most clinical triallists come from an academic background, but it should be seen as a career path for GPs and even specialists.”

Several of the world’s most important TB clinical trials of the past two decades were done in part or entirely in South Africa. (Photo: Rosetta Msimango / Spotlight)
Dheda says we are not training enough scientists and clinician scientists to undertake clinical trials and also highlights the lack of a fixed career path for academic scientists and clinicians.
“The MRC [Medical Research Council] must be credited for taking steps in the right direction by providing funding for postdoctoral training and for training clinician scientists, but a much more comprehensive and structured programme is required,” he says.
Dheda adds that South Africa needs information and solutions so that we can solve our own clinical problems, including those relevant to TB and HIV.
“It is also important to support and drive a knowledge-based economy, which is a major strategic objective of organisations such as the Department of Science and Technology and the [Medical Research Council]. For example, we are unsure about exactly how much TB costs the country, but it’s been estimated that it reduces our GDP by about 3%, not taking into account the chronic disease associated with TB and also not taking into account HIV. Put together (TB and HIV), this represents a huge negative impact on our GDP in the region of about 4% to 5% which is far more than our entire health spend,” he says. Therefore it makes sense for us to invest in research infrastructure that will generate solutions for our country and for our people.
“We are simply not investing enough into an exercise that will be extremely cost-effective.” DM
This article was produced by Spotlight – in-depth public interest health journalism.
Note: The Bill and Melinda Gates Foundation is mentioned in this article. Spotlight receives funding from the foundation. Spotlight is editorially independent and a member of the South African Press Council.






















 Become an Insider
Become an Insider
Great article!
Are there other countries that could provide a model for convincing talent in the medical field to pursue a career as a clinician?
Also, I am not sure that Prof. Dheda’s claim is correct that a “negative impact on our GDP in the region of about 4% to 5%” is greater than SA’s total healthcare spend (~8.5 percent per UNICEF). Was he referring to government spending on healthcare (~4 percent)?