
ADDRESSING OBESITY
Emerging research highlights the impact of gut microbiome on overall health

As the world grapples with an obesity epidemic, increasingly in developing countries, policymakers’ understanding of what drives obesity — which, in turn, increases the risks for a range of life-threatening diseases — has become a public health priority.
Scientific research into the gut microbiome and its effects on our overall health is still in its infancy, but there is growing evidence that the composition of our unique gut microbiomes may have wide-ranging influences on our bodies’ “energy balance” — how much energy we absorb from our food, how our fat cells behave, and whether we are predisposed to becoming overweight or obese.
As the world grapples with an obesity epidemic, increasingly in developing countries, including South Africa, policymakers’ understanding of what drives obesity — which in turn increases risks for a range of life-threatening diseases — has become a public health priority.
In South Africa, 13% of children under five are overweight or obese, increasing to 40% among adolescent girls and 68% among adult women. This obesity burden, which is a form of malnutrition, is driving a fast-growing epidemic of non-communicable diseases such as type 2 diabetes, hypertension, cardiovascular diseases and some cancers.
Until recently, public-health nutrition policy has focused on “macronutrients” — protein, carbohydrates, fats — and how these affect our digestion, metabolism and weight loss or gain. In the past few years, scientists focusing on obesity have begun to discover what seem to be astonishing links between our gut microbiome (or “gut microbiota”, or “gut flora”) and other bodily (and brain) functions.
For now, much of the research is tentative in its claims, simply because the idea that the gut microbiome affects metabolism and weight control is new, and consensus in the scientific community needs to be built through more clinical trials testing the same thing to corroborate these early results.
There will certainly be an ongoing stream of research in years to come that will delve deeper into this concept and reveal more precisely the mechanisms behind these links. Among these are links between the chemicals and additives widely used in ultra-processed foods that have been shown to be harmful to our gut microbiome, causing addiction and overeating, and thus driving weight gain.
Daily Maverick will continue to report on new research in this field. Here are a few recent highlights from the most recent gut-microbiome research to emerge in the past six months.
Gut microbiome is ‘a control centre’ for our energy balance
The eminent academic journal Nature recently published a small but possibly game-changing study by scientists from across the US showing how a “microbiome enhancing diet” containing mainly whole and largely unprocessed foods, compared to a “Western diet” featuring high levels of processed and ultra-processed foods, results in excretion of more energy, in the order of 5-10% of daily energy intake.
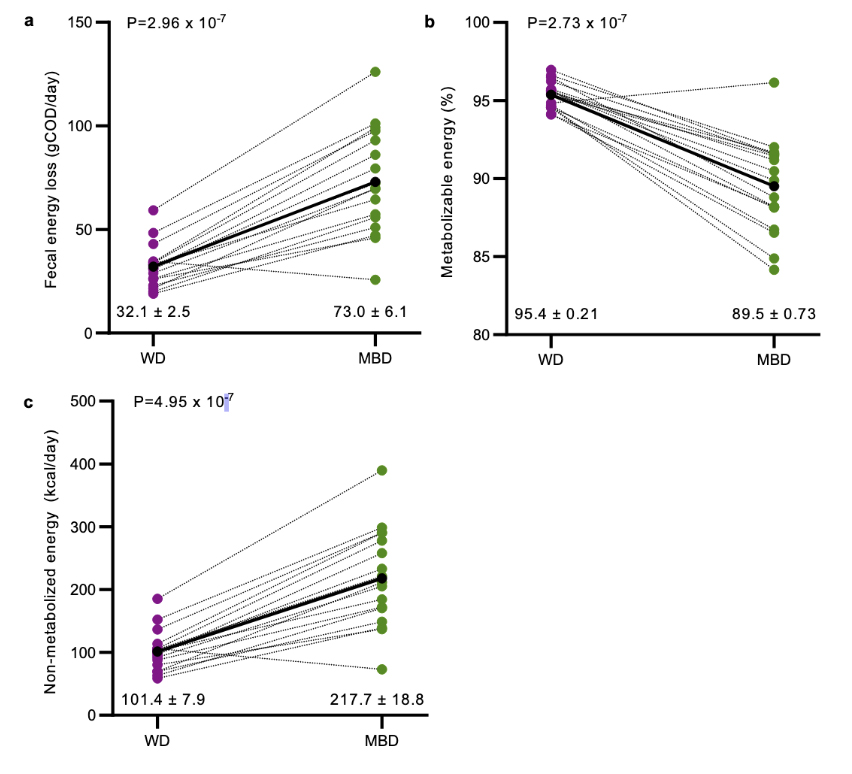
These graphs from the Nature gut microbiome study show how for 16 out of 17 clinical-trial participants, the ‘microbiome enhancer diet’ increased faecal energy loss (graph a), decreased the amount of energy available to be absorbed by the body (graph b) and increased the amount of non-metabolisable energy (graph c).
The clinical trial they conducted was thorough and intricate, investigating how the gut microbiome affects various aspects such as “energy harvest” from food, hormone regulation and the influence of metabolites generated during digestion on our body’s energy management of absorbed nutrients.
While previous studies have shown that high-fibre diets are associated with lower energy absorption from food, they lacked precision about the contribution of the gut microbiome to “the entire energy balance equation” — including energy consumed, energy expended, and how much energy left our bodies, unabsorbed. The researchers also said that earlier studies were not precise enough in detecting small differences in individuals’ gut microbiome compositions — which is a critical factor, given that each of our microbiomes is unique, having evolved since birth.
This trial compared participants eating a “highly digestible” Western diet (WD), which included a lot of processed, low-fibre, easy-to-digest foods, and a “microbiome enhancer diet” (MBD) that emphasised dietary fibre, resistant starch, large food particle size and limited processed foods.
The trial’s main finding was that the MBD resulted in “a significant decrease” in the amount of energy metabolised by 16 of the 17 trial participants, measured by how much energy they excreted (there were complex controls monitoring each individual’s energy balance).
The study then looked at the type of gut bacteria associated with each individual’s energy balance, and found (among other things) that on the microbiome enhancing diet, more energy went to the gut microbes (instead of being absorbed by the rest of the body), and there was more fermentation in the microbes (a good thing). On the Western diet, the gut microbes were “starved” because more of the energy available in those foods had already been digested and absorbed in the upper gastrointestinal tract.
Ultimately, the lower amount of metabolised energy was due to “increased faecal energy output” (calories excreted in the stool). This was not due only to undigested food, the study found, but also to the increase in fermenting gut microbes and their metabolites (substances that are made when the body breaks down food or tissues within the body). The amount of this energy loss through excretion translated to 116 kilocalories per day when participants were on the MBD. (The researchers modelled this to be the equivalent of about 200kcal/day equivalent reduction in food intake.)
This study, the researchers say, “demonstrates the potential to enact the ‘small changes’ principle” — the promotion of small changes in energy intake and expenditure — which “could be a useful population-level tool to fight the global obesity epidemic” through the consumption of whole foods to modulate the gut microbiome.
It also suggests that “an intentional remodelling” of the gut microbiome through a diet that has enough whole, naturally occurring fibre, resistant starch (from certain carbohydrates cooked and then cooled before eating), and “a focus on whole, minimally processed foods” resets the body’s complex mechanisms affecting food intake and our bodies’ stores of energy.
Bottom line? A microbiome-friendly diet may result in less caloric absorption than a typical Western diet, making weight loss and maintaining a healthy weight much easier.
Toxins in the gut change how fat cells function
Scientists from Nottingham Trent University in the United Kingdom have found that fragments of bacteria in the gut, known as “endotoxins”, damage fat cells and can drive weight gain, The Guardian newspaper reported. The researchers published their findings in the journal BioMed Central in May 2023.
The researchers’ aim was to understand the possible role of endotoxins in increasing someone’s risk for obesity or type 2 diabetes. Endotoxins are toxic bacteria normally present in the cell walls of bacteria in the gut lining, which are released when the cell wall breaks. This is a normal part of bacteria’s life cycle. People with obesity have higher levels of endotoxins, and their gut barrier is more fragile, which allows endotoxins to enter the bloodstream more easily.
Among the clinical trial group of 156 participants, 63 were classified as obese. The scientists examined two types of fat cells — white fat cells which store energy, and brown-like fat cells which use energy — and discovered that the white fat cells taken from people with obesity were less likely to turn themselves into brown-like fat cells. The reason for this, the researchers concluded, is because of the higher levels of endotoxins in the blood of obese individuals.
The roughly 38 trillion bacteria that dwell deep within your intestines are collectively known as the gut microbiota or gut microbiome. They help digest food, control hormones, and are increasingly being shown to function like a distinct organ in your body. (Credit: Harvard Health Publishing)
“It appears that as we gain weight, our fat stores are less able to limit the damage that gut microbe fragments may cause to fat cells,” Professor Mark Christian, the lead researcher, told The Guardian. “Our study highlights the importance of the gut and fat as critical interlinked organs that influence our metabolic health.”
Toddlers’ gut bacteria may predict future obesity
Gaël Toubon, a researcher from the Université Sorbonne Paris Nord, has found a “positive association” between obesity in five-year-olds (measured by body mass index scores), and their ratio of two types of gut bacteria that are directly related to obesity (Firmicutes to Bacteroides) taken from their stool samples when they were three-and-a-half.
In general, the higher the level of Bacteroidetes, the less likely it is for an individual to be obese. These bacteria are among trillions of others that make up the gut microbiome in every human that grows and develops in babies’ early months and years.
In 2006, researchers from Washington University School of Medicine in St Louis, Missouri, established the impact on body weight of the ratio of Firmicutes to Bacteroides bacteria present in the intestinal tracts of mice.
Toubon used data from 512 children, taken during a study that tracked the lives of 18,000 children born in France. The results were presented at the European Congress on Obesity in May 2023, in Dublin, Ireland.
“The reason these gut bacteria affect weight is because they regulate how much fat we absorb,” Toubon explained. “Children with a higher ratio of Firmicutes to Bacteroidetes will absorb more calories and be more likely to gain weight.”
Overall, six types of gut bacteria were “highly predictive” of higher body mass index scores among the five-year-olds.
“The gut microbiota is emerging as an important early-life factor able to influence weight gain in childhood and later life,” Toubon said. “Our findings reveal how an imbalance in distinct bacterial groups may play an important role in the development of obesity.”
Toubon said further research was needed to understand in more detail which specific bacterial species influence the risk of and protection from obesity, and when the switch to an obesity-favourable gut microbiota may take place, to better understand the right timing in an individual’s life-course to make the right interventions. DM





















 Become an Insider
Become an Insider
Comments - Please login in order to comment.