BHEKISISA OP-ED
How Covid sped up South Africa’s medicine approvals process
During the Covid-19 pandemic, the South African Health Products Regulatory Authority (Sahpra) was forced to speed up its review of new medicines such as vaccines, while still ensuring that they were safe and effective.
A leap year comes around every four years. As does the Olympics. And in the past, probably an approval of a new medicine by the South African Health Products Regulatory Authority (Sahpra).
But the Covid pandemic shook things up — and for the better in the case of getting Sahpra-approved medicines and other health products such as diagnostic tests on to the market.
Before Covid, it could take Sahpra three to four years to approve a new product because the regulator was steadily working through a huge backlog of applications. This was because, at the time, rules for medicine approvals required us to prioritise applications for products that contain an ingredient that’s on the government’s essential medicines list. And because there are many generic versions of such products too, the number of applications that needed to be dealt with piled up. The rules have since been amended so this condition no longer applies.
During the pandemic, though, we were able to cut the review process of Covid-19 vaccines down to between four and 11 months — on average, five times faster than before — without compromising our assessment standards. Moreover, during this time, we continued to also prioritise approvals of products such as HIV prevention medicines, antiretrovirals for HIV, and medicine for TB and cancer (this process was in place before Covid), in addition to Covid treatments and vaccines.
How? By streamlining how we work.
A do-or-die moment
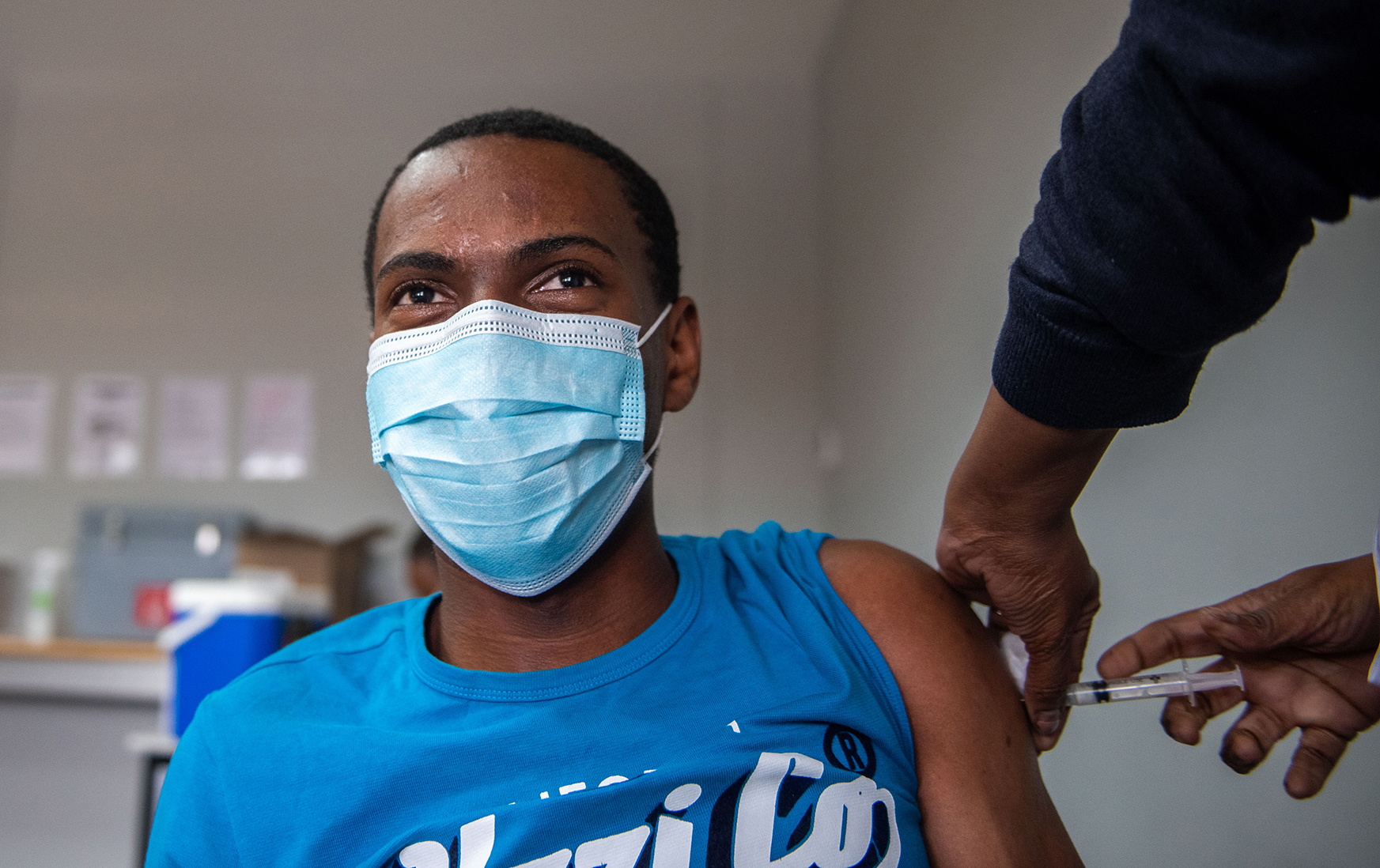
In the case of Covid vaccines, emergency authorisations helped to get lots of people vaccinated fast. (Photo: Alet Pretorius)
The pandemic was a do-or-die moment at Sahpra. Because of the public health emergency, many more applications landed on our desks than before, on top of the long backlist of applications — but our staff numbers stayed the same. This pushed us to work smarter, so that our processes would not only speed up but also improve.
The lessons we learnt will benefit everyone in South Africa in the long term, because more efficient processes mean that people won’t have to wait unnecessarily long for new medicines that come on to the market because of administrative wheels turning slowly.
But our new processes also have wider benefits.
Having strong regulatory systems in place is part of achieving universal health coverage, says the World Health Organization (WHO) because it reduces the chance, especially in developing countries, that people will buy counterfeit, unsafe or ineffective medicines.
Universal health coverage means that all people, regardless of how much they’re able to pay, get the health services they need. This type of coverage is part of the United Nations Sustainable Development Goals, which 193 countries, including South Africa, have agreed to achieve by 2030.
The plan with which South Africa aims to get universal health coverage is the National Health Insurance (NHI) scheme. Many details about the NHI still have to be decided on and the NHI Bill has been widely criticised for gaps.
One way to address at least some of these shortcomings is through Sahpra’s faster approval processes.
Shaking up Sahpra’s systems has paid off
The WHO has a Global Benchmarking Tool that specifies what strict systems for overseeing the safety, efficacy and quality of health products should look like, to help countries’ medicines regulators improve. There are four maturity level ratings, ranging from a country having some elements of a control mechanism (Level 1) to the regulatory system being highly developed and improving all the time (Level 4).
Shaking up our systems has already paid off.
In October, the WHO awarded Sahpra’s vaccine reviewing system Level 3 maturity. This means that all the processes needed to ensure that people get safe immunisations — from bookkeeping to ensuring quality production processes to overseeing the details of how clinical trials are run and keeping records of side effects and what caused them — can be trusted to work well.
Moreover, Sahpra’s systems for quality-checking each lot of vaccines before distribution are certified to work at Level 4 standards. (A lot is a large batch of products all produced at the same time at a single facility.) A Level 4 standard means Sahpra is operating at an advanced level and continuously improving its processes.

The hub is a project the World Health Organization set up in 2021 to help poorer countries to make their own vaccines. (Photo: Aspen Pharmacare)
This is good news for the mRNA vaccine technology transfer hub in Cape Town, because it means people can have faith that the jabs developed there are up to international standards for use.
The hub is a project the WHO set up in 2021 to help poorer countries to make their own vaccines.
For other functions, for which Sahpra is currently graded at Level 3, we’re planning to reach Level 4 maturity by 2025. That will put us on par with authorities listed as “stringent regulators”, such as those in the United States and the United Kingdom.
Here’s how things used to be done — and how we’re speeding them up.
How are medicines approved?
Medicines can be authorised for use in South Africa based on four types of approvals.
- Full registration: This process takes at least a year, Sahpra’s 2020 performance plan For this type of approval, the documents submitted by a drug manufacturer to apply for registration go through a two-step screening process to make sure that only applications that meet all the necessary specifications enter the formal review system.
Two evaluators work through all the data about the medicine gained during clinical trials. One person is the main reviewer and their partner double-checks the details of the application. If both assessors agree that there isn’t any further information needed from the manufacturer (for example, about quality, how the drug works compared with another similar one, or the production process), they pass the application on for quality control conducted by a different team. If the evaluation process reveals any queries that would need more discussion, the application is passed on to a technical advisory committee.
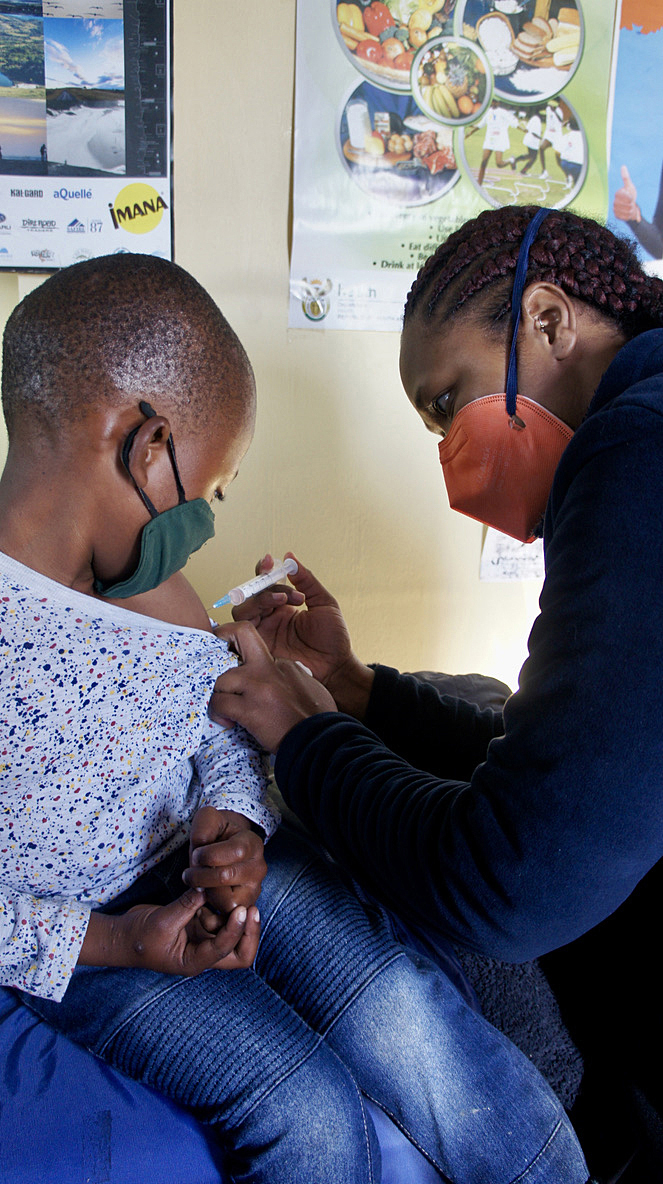
In October, the World Health Organization awarded the South African Health Products Regulatory Authority’s vaccine reviewing system a level three maturity. This means that all the processes needed to ensure that people will get safe immunisations are in place. (Photo: Sigrid Kite)
Once those issues have been addressed by the manufacturer, Sahpra will decide whether the medicine should be fully registered for use in South Africa.
- Conditional registration: Sahpra first used this category during Covid and specifically for vaccines, but it’s something we’ll use for any future public health emergency.
It works like this: If there’s enough evidence that a medicine or other health product is safe, works as it should and meets quality standards, Sahpra will allow the drug or product to be used by the public while the manufacturer waits for full approval. This can be helpful when research is still under way and the full data set that would usually have to be presented, is not yet available. It’s not a free pass, though: new, detailed information must be submitted within three months to a year, depending on the terms of the conditional approval.
- Section 21 authorisation: This type of authorisation can be granted if a medicine usually prescribed for a condition won’t work for a specific patient or if it’s not available, and an alternative needs to be used. If the application is approved, a doctor would be allowed to order the substitute, despite it not being registered in the country. The company that imports the medicine must, however, be licensed by Sahpra.
Doctors would normally have to apply for their patients to be treated with the medicine individually, but ‘bulk’ approvals can also be granted when more than one patient needs the unregistered medicine.
Instead of the process taking a year or more, as for full approvals, a Section 21 application can be processed in three days or less. The catch is that this type of approval lasts only for six months.
- Public health emergency authorisation: Medicines needed for dealing with a public health emergency (such as Covid) can get into the approval system either as Section 21 or conditional applications. The process for getting the medicine approved can start — and be completed within two to six weeks, given that all the required information is made available. A benefit-risk assessment is done for each product that is reviewed.
Emergency use authorisation lasts only for six months.
In the case of Covid vaccines, emergency authorisations helped to get many people vaccinated quickly, because they could get a shot such as Pfizer/BioNTech’s jab Comirnaty before it was granted full approval by Sahpra. (Cominarty got conditional approval only in January 2022, although by then about nine million people in South Africa had had two shots of that vaccine.)
We learnt three important lessons from implementing these types of approvals.
Lesson 1: One meeting can be worth a thousand emails
During Covid, we set up a meeting with the drug manufacturer before it even started its application process.
In this discussion, the pharmaceutical company could share what information it already had available, such as data from laboratory tests, initial safety or quality information or early results from clinical trials. The company could then ask us to clarify points in the application process it was unsure of. In turn, we could alert it to gaps in its data or about extra information we would need.
This significantly cuts down on the usual back-and-forth, because when the paperwork is eventually submitted, the regulator has had a heads-up about what’s coming and the information the evaluators get to work with is prepared better. This means we’ll have fewer questions for the applicant, so the review can go quicker.
We’re planning on making these meetings an option for any application in the future.
Lesson 2: Eat an elephant one bite at a time — rolling reviews help
During the pandemic, a process called “rolling review” allowed Sahpra’s team to analyse a product’s data as they became available instead of having to wait for everything to be ready and then having to work through a massive amount of information all at once. Regulators in other countries had a similar system.
It was used for the Johnson & Johnson Covid vaccines used in South Africa, for instance. The manufacturer applied for registration, but submitted the supporting data to us in batches as they became available. Although clinical trials were still under way when the pharma company initially applied, getting some data earlier gave us a head start.

A public hearing on the National Health Insurance in Kagiso Johannesburg. (Photo: Rosetta Msimango / Spotlight)
Doing rolling reviews also halves the paperwork, because a company submits only one application for registration of its product, instead of putting in a Section 21 request first and then later going through another application round.
Our Covid experience showed that rolling submissions can significantly reduce the time it takes to get medication to patients, especially when there’s an urgent public health need. Faster access could help the NHI scheme deliver universal health coverage.
Lesson 3: Sharing is caring — especially in a health emergency
Sahpra is part of a network of medicines regulators around the world. Being able to use “reliance mechanisms” during the Covid pandemic helped us to do our own reviews faster. For example, when one of the internationally regarded trustworthy authorities — such as those classified as stringent regulators or others vetted by the WHO — has already approved a vaccine, we could use their analyses and reports to inform our decisions.
The challenge with this approach, though, is to get other countries’ regulators to share their reports without delay, which means that we have to maintain good relationships with such partners. In some cases, this strategy requires signing a memorandum of understanding or a non-disclosure agreement with a partnering regulator, as Sahpra has now set up with, among others, the US Food and Drug Administration (FDA). This means that we’ll be able to use these regulators’ reports and analyses for any product application that has already passed their desks — which can help us to get medicines to people faster.
With these changes in place, we believe Sahpra can be a stronger partner for the NHI to ensure universal access to healthcare in South Africa. DM/MC
Portia Nkambule is the chief regulatory officer of the South African Health Products Regulatory Authority. She’s a pharmacist with a master’s degree in public health

"Information pertaining to Covid-19, vaccines, how to control the spread of the virus and potential treatments is ever-changing. Under the South African Disaster Management Act Regulation 11(5)(c) it is prohibited to publish information through any medium with the intention to deceive people on government measures to address COVID-19. We are therefore disabling the comment section on this article in order to protect both the commenting member and ourselves from potential liability. Should you have additional information that you think we should know, please email [email protected]"




