SNOW WHITE’S TOXIC DWARFS (PART THREE)
Skin-lightening whitewash: Global and domestic bans of cosmetics containing mercury prove to be skin-deep
To protect people and the environment from the well-documented toxic harms caused by mercury, more than 130 members of the Minamata Convention agreed to a global ban on the manufacture and trade of cosmetic products containing more than 1 part per million of mercury from January 2021.
South Africa banned the use of this brain-poisoning heavy metal in cosmetics more than 30 years ago. Yet, skin-lightening creams with very high levels of mercury and other dangerous substances are still sold openly across the country — not just in shops or from informal vendors, but also over the internet through e-trading platforms such as Facebook, eBay, Desertcart, Takealot or Gumtree.
Clearly, the global and domestic bans aren’t working, exposing millions of South African women, men and children to a variety of significant health risks.
But with so many agencies and other roleplayers involved directly or indirectly in enforcing bans or monitoring illegal activities, who is to blame and what needs to be done?
To answer some of these questions, we went on an unusual shopping expedition just before Christmas, to see how easy it is to buy some of these skin-lightening products.
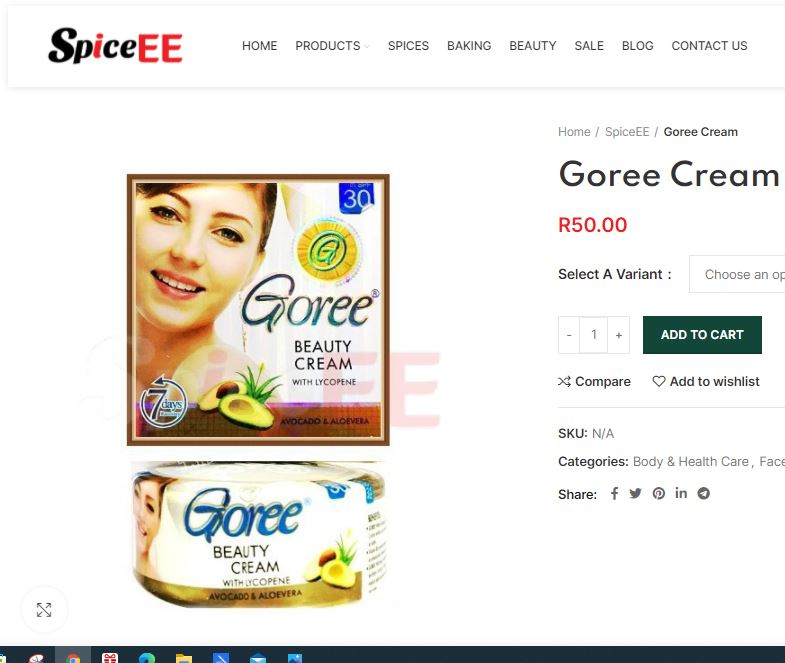
Based on previous research by the local environmental justice watchdog groundWork, we bought six different skin-lightening creams — one from a SpiceEE branch in Pandora Street, Phoenix; three from another SpiceEE branch in nearby Parthenon Street and two from W&G Makeup City at Durban China City in Springfield Park. All six products were made in Pakistan, according to the package markings.

The six skin-lightening creams analysed by Our Burning Planet. (Photo: Tony Carnie)
Then we sent the samples to CSIR Analytical Services in Stellenbosch for laboratory tests. The results showed that all six samples contained mercury levels above 1 part per million (ppm) — with one sample containing over 29 000 pm of mercury.
The full results are presented below:
Goree beauty cream with lycopene: 29,218 ppm
Golden Pearl beauty cream: 16,857 ppm
Goree Day and Night cream: 14,047 ppm
Faiza beauty cream: 5,890 ppm
Biocos beauty cream: 4,766 ppm
Noor Gold beauty cream: 82 ppm
Five of these six creams have also previously been tested at other laboratories in Greece and California and found to contain mercury levels varying between 9,000 ppm and 16,000 ppm.
We also sent questions to the national Department of Health about the apparent failures in regulation and the monitoring of domestic and imported skin-lightening creams.
The department was asked to explain why draft amendments to the Foodstuffs, Cosmetics and Disinfectants Act in December 2017 have still not been promulgated. The draft includes provisions to tighten up on the monitoring, control and sale of these products, but department spokesperson Foster Mohale has not responded to questions sent in December.
We also sent questions to the SA Health Products Regulatory Authority (Sahpra), an entity of the Department of Health tasked with regulating, monitoring, evaluating, investigating, inspecting and registering all health products.
“Why do the Sahpra and other sister agencies appear still to be failing to control the manufacture, distribution and use of skin-lightening creams containing illegal and harmful ingredients?”
Sahpra said: “There are sophisticated criminal networks operating on a global scale, not only locally. Sahpra coordinates with other law enforcement agencies in the prevention, detection and response to this challenge. Stakeholder cooperation and collaborative efforts are being strengthened in this regard.”
We also asked for an update on the seizure of almost R1.50million worth of skin-lightening creams and medicines in Cape Town by Sahpra and other government agencies in December 2019. Where did the products come from? Was anyone convicted?
The authority suggested we contact the SA Police Service (SAPS) for more information on this, but noted that in another operation in Johannesburg in November 2021, the authority worked with Interpol and other government agencies, leading to the seizure of “thousands of such products” and the arrest of another two suspects.
On whether Sahpra has any current data on the volume of skin-lightening creams manufactured locally or imported on an annual basis, the authority said: “Due to false declaration of these substances at the ports of entry by importers, it was not easy to readily quantify/value this.
“However, there are other strategies and mechanisms being deployed in this area to prevent, detect and stop these (products) and other means still being explored.
“Sahpra is working hard in its market surveillance activities and these products are in the priority list of our annual targeted market surveillance of the health products supply chain. We urge the public or health professionals to report these to Sahpra.”
What about e-commerce and online sales direct to the public? How is this policed?
“Sahpra relies on stakeholders such as SAPS and SARS [SA Revenue Service] to monitor online sales or illegal imported products. Once the online sale of illegal medicine is detected, SAPS will investigate and Sahpra will play a supporting role. If the evidence is sufficient, the matter will be referred to NPA for prosecution,” the authority said, adding that many such creams appear to be coming from the Ivory Coast, India and Democratic Republic of Congo.
We also contacted the Customs and Excise division of SARS to find out what they do to monitor and inspect consignments of illegal skin-lightening creams.
According to SARS, customs officers conduct scheduled and unscheduled search and detection operations at all 51 ports of entry.
“Medicine, medicaments, and supplements are considered high-risk imports and the SARS Customs Risk system will provide an alert to customs officers for scheduled searches and inspections to be conducted. Risk-driven inspections are complimented by ad-hoc, unannounced manual inspections focussing on high-risk commodities including medicaments.
“SARS Customs forms part of the whole-of-government approach to prevent and detect legislative non-compliance and frequent inland joint operations are conducted to detect suspected illegally imported illicit/illegal goods including counterfeited goods, medicaments, clothing, textile, leather, footwear, cigarettes, tobacco products, alcohol, narcotics, endangered species, firearms, and ammunition to mention a few.”
What happens when dodgy shipments of skin-lightening creams are seized? Do SARS officials have the training and access to accredited laboratories at border posts to rapidly analyse these creams for mercury and other harmful substances?
The division says such products are handed over to the Department of Health for verification and eventual destruction, or “referred to the local Port Health Officers with the technical knowledge for assessment and a decision”.
To monitor e-commerce and online purchases of imported skin-lightening creams, the division says inspections are carried out at international mail centres, courier import companies, along with traveller luggage inspections, commercial and non-commercial cargo and container inspections and the use of scanners. SARS said it was not able to provide any data on the overall volumes / monetary value of illegal skin-lightening creams seized by customs officials over the past calendar year.
However, over the last year, the division had seized 347 boxes of Betanol, Diprosone Epiderm Lotion and Carolight skin products concealed in bran. These were handed over to SARS Customs by the SANDF in December 2021 at Komatipoort. At the Lebombo Border post, 60,084 units of White Secret skin cream and several vitamin products were seized in September 2021.
SARS also has a significantly different list of countries linked to illegal skin creams, suggesting that most creams come from Pakistan; the Middle Eastern Countries and the Caribbean. It also said that Mozambique, Zimbabwe and Botswana are significant transit countries for such products.
Despite the 1990 domestic ban on the sale and marketing of harmful skin-lightening products, it remains unclear what proportion of these products are still made in South Africa.
A research paper published by Durban dermatologist Prof Ncoza Dlova in 2012 suggests that significant volumes of illegal skin bleaching creams are still made locally. She said an investigation into the top 10 bestselling skin-lightening creams available in Durban indicated that 90% of creams sampled were found to contain banned or illegal compounds.

Golden Pearl advert. (Image: Supplied)
Nearly 60% were manufactured in South Africa and the rest were imported illegally from Taiwan, Italy and the UK. Nearly 40% of the analysed creams contained mercury as an active ingredient, 20% contained corticosteroids, 20% contained resorcinol and 10% contained a derivative of hydroquinone.
“Of major concern also is the fact that prescription drugs such as corticosteroid creams are easily dispensed by some unscrupulous pharmacies without an official prescription, and are sold at some general practitioner rooms by receptionists without the patient consulting the doctor,” she reported.
Hoping to get some answers about the domestic manufacture of skin-lightening creams, we contacted Adelia Pimental, executive director of the Cosmetic, Toiletry and Fragrance Association of South Africa (CTFA) an industry lobby group established in 1994.
The association says it endorses Government Regulation R1227 (1988), which specifically prohibits the use of mercury in cosmetic products. It also has a position statement.
Do any CTFA members distribute locally made or imported skin-lightening products over the counter? If so, what are the lightening agents used in these products?
Pimental says there are many CTFA members that make skincare products, including some brands with “even skin” product ranges.
“The main function of these products is to even the natural tone of the skin, rather than lightening it per se, and there are many ingredients which provide that specific benefit which have a clinically proven safety profile for consumer use.”
Does CTFA have any information on the current size of SL products in South Africa in terms of annual market value? Is it growing or declining?
“The CTFA’s focus is to ensure that safe and effective cosmetic products are placed on to the South African marketplace, rather than record market statistics for individual product categories,” Pimental suggests.
Does CTFA have a complaints and disciplinary codes of practice for members who add mercury to skin creams? If so, what is this code, and what are the sanctions in relation to the use of mercury/hydroquinone in SL products?
“The CTFA is a non-statutory professional trade association and as such members are solely responsible for their behaviours. Sanctions and fines can only be issued by the regulator which, in this case, is the National Department of Health.”
Since the establishment of the CTFA in 1994, has any member ever been found to have contravened the association’s code of practice or standards in relation to the use of mercury/lead/hydroquinone or other potentially dangerous substances used in SL products?
“CTFA is not aware of any member that has been found to be utilising mercury, lead or hydroquinone in any cosmetic preparation that falls under the ambit of Government Regulation R1227.”
We also asked the association if it was concerned about stricter regulations for labelling, advertising and composition of cosmetics proposed by the government in December 2017 and whether CTFA could throw any light on why there had been a lengthy delay in promulgating them?
“Despite the National Department of Health’s original intention of promulgating these regulations in 2017, this has not occurred to date. It is not for the CTFA to comment on the reasons for the delay in promulgation of the draft regulations, as this falls wholly under the ambit of National Department of Health.”
Is CTFA concerned that these proposed regulations could undermine the self-regulatory nature of the cosmetics industry?
“Not at all,” says Pimental. “In fact, on the contrary, these regulations will be welcomed by the CTFA, as they will only serve to create further credibility of the cosmetic industry in South Africa. CTFA has been a massive contributor of the regulations to the NDOH.”
We also asked the association to comment on criticism by University of KwaZulu-Natal law student and researcher Nabeela Seedat about the self-regulatory nature of the CTFA. Seedat suggested in a recent dissertation that consumers were not able to access the association’s codes of practice or to lay complaints and suggested that the failure of the CTFA to regulate the industry was one of the reasons that stricter government regulation was necessary.
“Ms Seedat’s allegations are somewhat misguided, in that the CTFA was never intended to provide a forum for consumer complaints. Indeed, there are many forums via which consumers may complain about any cosmetic product, including the Advertising Regulatory Board of South Africa, the Consumer Protection Act and the National Department of Health, depending upon the nature of the consumer complaint concerned. Furthermore, CTFA has been insisting for a long time for the cosmetics industry to be regulated, in order to protect consumers more. CTFA has been lobbying for the promulgation of the regulations for a very long time.”

A Golden Pearl whitening serum advert from the Golden Pearl website. (Image: Supplied)
“The allegation that government regulation was introduced because of the CTFA’s lack of ability to regulate cosmetic products in South Africa is without foundation, as the sale of cosmetic products in South Africa has effectively been governed by the Foodstuffs, Disinfectant and Cosmetic Act (Act 54) since its publication in 1972, when the CTFA was only established in 1994.
“In contrast to Ms Seedat’s allegation, the CTFA has been highly instrumental in the co-development of the Draft Foodstuffs, Cosmetics and Disinfectants Act published in December 2017, which is still to be promulgated through parliament . . . The CTFA was and still is, aligned with these regulations, so it has no cause or reason to delay promulgation in any way.”
One senior government official we spoke to believes that the time has come for much stricter penalties to deter the purveyors of harmful SL creams.
The official, who is not authorised to speak to the media and cannot be named, said: “There is still a big gap in legislation. Current fines are in the region of R10,000.”
Because of this, the National Prosecuting Authority was reluctant to assign prosecutors to cases where fines are so low. The official also points to several other constraints in enforcing existing legislation.
Very few departments set aside annual budgets for chemical analysis of potentially hazardous goods or their storage and subsequent disposal in toxic waste facilities.
There were also no laboratory facilities at border posts for rapid, independent analysis (even for illegal drugs), so samples had to be sent to third-party laboratories — if sister agencies had the budget. Often, the customs department was left holding seized goods because there were no dedicated financial resources to analyse or dispose of them safely.
Because penalties were so low for cosmetics-related offences, customs officials sometimes opted to prosecute offenders for their failure to declare goods or for misdeclaration of goods under the Customs and Excise Act (which provides for jail terms of up to five years for such offences).
Prof Dlova, who has been campaigning to raise public awareness about harmful skin lightening creams for several years, notes that South Africa’s borders remain “porous”.

Professor Ncoza Dlova leads a protest against skin-lightening creams in Durban. (Photo: Nombuso Dlamini)
“When one engages the relevant authorities in the relevant departments, they often have other priorities, or the interventions are not sustained or monitored. But I must say the Department of Health has been quite supportive in advocating against the abuse of such creams and there have been raids in some parts of the country.”
Another complication, she says, is that the distributors often change their product names after enforcement drives.
“I think the most important step is to educate consumers from a young age so that they are properly informed, because one can’t rely on government to regulate this problem when they have other pressing priorities. While some of the products are manufactured in other countries, I would say about 70% are manufactured right here in SA, and that is what our studies revealed.”
“The government also needs to be consistent in monitoring, conducting spot-checks and raids on the vendors. It has to be ongoing — not just once a year.”
Dlova also believes health workers have an important role to play in educating and discouraging patients from using these creams … Most patients still don’t know that there are [harmful] side effects.” DM
Responses from manufacturers and sellers
We were not able to reach SpiceEE owner Ali Osman for comment on the CSIR laboratory test results. His father, Ebrahim, initially asserted that “those creams have been discontinued” before remarking “I am not the only one selling those creams … If we were aware that it was illegal, do you think we would put it online?”
Asked if he knew that the sale of skin creams containing mercury had been banned since at least 1988, Osman senior said: “Not to my knowledge. I don’t know the content of these creams. My knowledge is limited to spices. How do they (creams) come into the country if they are illegal?”
We asked for a contact number for Ali Osman, but his father said: “He is not online or on the phone. He has taken a two-week vacation to the Berg.”
Nor were we able to contact W&G Makeup City on the cell phone number listed on the receipt and shopping bag.
Arslan Tariq, spokesperson for Goree Cosmetics in Lahore, Pakistan, asserted that the creams we tested must have been fake.
“As per our survey there are too much duplicate / copies of our Goree products In the market. As we do not operate directly in the SA market some people take advantage of that and by using our name they throw fake Goree products in the market in fact many of them are not even in our product range but they are selling under our name in South Africa.
“We are totally aware about it should be > 1ppm mercury and we assure you that we do obligate that. (Samples can be provided on demand). If you guys have any office in Pakistan you can take Goree cream from market here in Pakistan or tell us can send samples to you. So that you can be able to share the real picture even to our customers too.”
This is not the first time Goree has responded in this way to tests in other parts of the world which also show high levels of mercury in Goree brand products.
Noor Gold spokesperson Muhammad Kashif offered a similar response to our queries: “Our products are according to international quality standards. And does not contain hazards contents. But some people make copy of our products for making big profit.”
Golden Pearl, Biocos and Poonia Brothers (Faiza cream) did not respond to email queries. DM
Disclosure and disclaimer: This series of articles was commissioned by groundWork to raise public awareness about the dangers of mercury and toxic skin-lightening creams. groundWork acknowledges financial support by the Swedish public development co-operation aid through the Swedish Society for Nature Conservation and the European Commission via the European Environmental Bureau. The sole responsibility for the content of the reports lies with groundWork/Daily Maverick. The funders are not responsible for any use that may be made of information contained therein.
















 Become an Insider
Become an Insider
Comments - Please login in order to comment.