NEWSFLASH
Ivermectin and Covid-19: SA drug regulator allows controlled, compassionate access
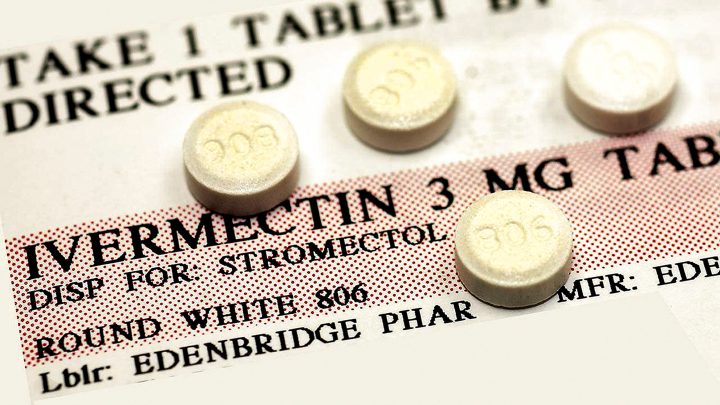
South Africa’s medicines regulator finds middle ground to authorise ‘compassionate use’ of ivermectin, whilst still stating that there is not yet sufficient evidence for full registration.
This afternoon Boitumelo Semete-Makokotlela, CEO of the South African Health Products Regulatory Authority (SAHPRA) announced that it would “facilitate a controlled compassionate access programme” to the drug ivermectin for use for patients with Covid-19.
“Very detailed guidelines of this programme” will be announced “in coming days” that would build in the principles that are used to inform and grant s21 authorisations under the Medicines Act and would be “open to all patients”. A s21 authorisation is a mechanism used by SAHPRA for medicines that are not registered in South Africa, but which are known to be safe and efficacious and in use in other countries. In the past, how and when these authorisations have been granted has itself been a bone of contention, for example, overuse of generic drugs for cancer or HIV whose originals are excessively priced.
According to Semete-Makokotlela, applications would need to be made by health professionals so as to ensure they took responsibility for safety and monitor efficacy. “There’s a large responsibility we are placing on health professionals,” she said.
The drugs used as part of the programme would need to be drugs manufactured for human use (not animal use) and, given that there is no local production of the drug, it would have to be imported by a reputable company.”
During the briefing, Semete-Makokotlela, as well as SAHPRA Board chairperson Prof Helen Rees, were at pains to stress that since their statement on ivermectin January 6th 2021 they had “reviewed extensively the new data that has emerged, but that after careful and thorough consultations there is still, at this point, “insufficient data for the regulator to make a decision” on full registration of the drug for human use in relation to Covid-19.
Their consultation, Semete-Makokotlela explained, had included the World Health Organisation (WHO), other drug regulatory authorities, as well as the recently published meta-analyses conducted by researchers such as Prof Andrew Hill. This was the shared view of SAHPRA, the National Essential Medicines Committee and the Ministerial Advisory Committee.
However, most significantly, Rees was also at pains to stress that SAHPRA appreciated the urgency and emergency situation that faced doctors: “We are very sensitive,” she said, “we have listened and heard and understand the position health practitioners are coming from.” Some of these pleas by doctors have been made on the pages of Maverick Citizen — read Dr Freinkel here and Dr Mdladla here.
Rees also said that “we know the situation is desperate out there” and that “illegal use and importation was itself a safety concern that had to be taken into account. We are not able to control the dosage or quality [when ivermectin is used illegally], so until we get better data from imminent trials, let us allow compassionate use, but in a way that will also allow the regulator to monitor and collect data.” Rees said, “this will give us respite and assurance that the drugs being used are safe.”
In addition, Rees also said that SAHPRA would be looking at a longer-term controlled access trial of Ivermectin, as well as applications for clinical trials that are being submitted by universities — she mentioned: “three research groups whose applications are all being expedited.”
In the wake of today’s announcement doctors and patient advocacy groups expressed some relief. SAHPRA appears to have found a responsible middle ground that recognises the limited evidence but also the desperation and the fact that, even for a regulatory authority charged with rigidly ensuring medicine safety, quality and efficacy, flexibility may be warranted. In this respect, SAHPRA may be setting a precedent for other health authorities as well as helping garner evidence that will finally decide the efficacy of this medicine.
The one concern that is likely to be heard in coming days, is how fast SAHPRA moves to institute this programme and how it works with doctors to assist their applications. Even while there is acceptance that the evidence is incomplete, many would argue that for those severely ill with Covid-19 today, whether they get access this week or next week may literally be a matter of life and death. That is what health activists said they would continue to monitor. DM/MC
Information pertaining to Covid-19, vaccines, how to control the spread of the virus and potential treatments is ever-changing. Under the South African Disaster Management Act Regulation 11(5)(c) it is prohibited to publish information through any medium with the intention to deceive people on government measures to address COVID-19. We are therefore disabling the comment section on this article in order to protect both the commenting member and ourselves from potential liability. Should you have additional information that you think we should know, please email [email protected]
"Information pertaining to Covid-19, vaccines, how to control the spread of the virus and potential treatments is ever-changing. Under the South African Disaster Management Act Regulation 11(5)(c) it is prohibited to publish information through any medium with the intention to deceive people on government measures to address COVID-19. We are therefore disabling the comment section on this article in order to protect both the commenting member and ourselves from potential liability. Should you have additional information that you think we should know, please email [email protected]"



 Become an Insider
Become an Insider
Dear DM, our authorities are now paying attention! Not only as a treatment but preventative too,
an all out, super fast tracked conclusion is criminally delayed. Imagine the lives that could have been saved or the difference it could make to our economy if all can use it until vaccines become available. Best I say no more…😡
Ivermectin does not require a prescription. One used to be able to purchase these at your local Agrimark. I’m sure this is still case – surely? Or am I missing something? Just go out and buy your own…..?
Cath : that’s the animal product in liquid form
“The drugs used as part of the programme would need to be drugs manufactured for human use (not animal use) and, given that there is no local production of the drug, it would have to be imported by a reputable company.”
FYI – I have it on fairly good authority that the safe dose for human consumption is the 1 mg dose NOT the 3 mg. If you take 3 mg it could be dangerous.
Can anyone substantiate this please?
Well it is not to be ingested..its an animal dip! The form we get in South Africa is made for animals, to be absorbed through the skin and absorbed through fatty tissue. Ingesting it would, as I understand , make the effective ingredient go down the tube without efficacy, but all the other parts of the dip though the digestive system put huge pressure on your organs. What we need is a human dose form that is currently abundant and legal in the rest of our continent. Meanwhile, if you have the dip form, rub a bit on your tummy. That’s the pharmacological action you want.
I cannot see how a veterinary medicine used to kill parasites in animals can help with an entirely different viral illness in humans. SAHPRA have bowed to pressure. Next thing we’ll be eating garlic and dog biscuits as a cure !
Lets see what proper studies show before we allow human consumption.
It’s not only used as a veterinary product. It has been used to treat River Blindness in humans since the 1980’s (billions of doses). See the WHO for reference material. It just doesn’t happen to be registered for human use in South Africa. The responsibility lies with the pharmaceutical companies to register the product with SAHPRA. It’s dirt cheap with lots of generics so there isn’t a lot of incentive for pharmaceutical companies to register it, especially for a new “off-label” use.
It’s about pharmacological Action. From a long swing, Bill Bryson is a delightful author cos he can take this stuff and make it seem simply logical. And then we lay people understand. So he would use analogy and I guess in this instance he would say something like “it started out as an effective cure for parasites, but what they didn’t know for a whole 55 years is that this stuff also disrupted the xyz of the reproducing part of this damn virus which stopped the global economy… this teensy eensy little critter virus born of chinese parents in Wuhan. And then, like a lone soldier, a little pill, just doing its job getting rid of threadworms and river blindness in Africa suddenly had everything it needed to combat this global foe. And it did. The end.
@Wendy and @Alan. 100% agree. SAHPRA have opened the proverbial Pandora’s box:
– the drug is freely available and cheap: let’s use it. What science in that argument ?
– people are dying: let’s use it. We know that – again what science in that argument ?
– big drug companies are deliberately trying to supress the use of Invermectin – what science in that argument ?
SAHPRA’s brief is not “compassion” , nor championing of populist sentiment against “big drug companies”, nor (related) championing of cheap alternatives. SAHPRA’s brief is ensuring that vaccines’ and/or curative drugs are subjected to appropriately rigorous scientific trials. In that regard they have failed us. Despite massive political pressure in the US (eg on hydroxycholoraquine) , their FDA held firm against quackery and their advice on “Invermectin and Covid” should be mandatory reading for our authorities.
Actually the drug IS SAHPRA approved for human consumption!! These ANC clowns must get out of our way, this is life or death, not just how much more can they loot.
The NIH in US approved Ivermectin for off-label use in last few weeks.