SPOTLIGHT OP-ED
Despite significant challenges, South Africa’s drug regulator is on the right track
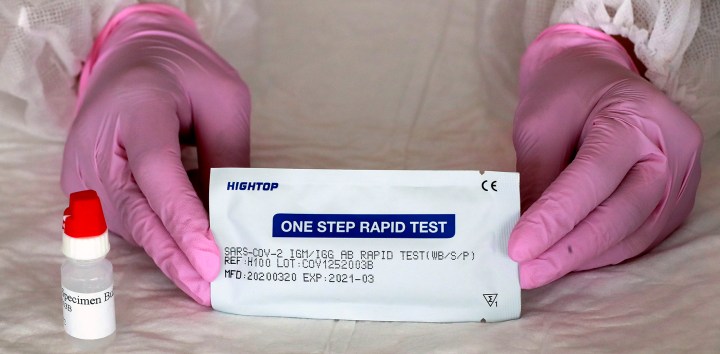
The South African Health Products Regulatory Authority was recently criticised for not approving laboratory-based SARS-CoV-2 antibody tests. Spotlight editor Marcus Low argues that despite the regulator’s capacity constraints, its flexible conservatism is something to be grateful for.
Regulators of medicines and other health products have a tough job at the best of times. On the one hand, industry and some patient groups are clamouring for products to be brought on to the market as quickly as possible; on the other, allowing an unsafe or ineffective medicine or product on to the market can cost lives or contribute to avoidable suffering or disability.
Finding the right balance between these competing interests is even harder amid the elevated pressures of a pandemic. Do you allow relatively inaccurate SARS-CoV-2 antibody tests on to the market, or do you protect the public from the consequences of these inaccuracies?
Where do you draw the line?
The good news is that over the past decade or so, South Africa’s health products regulator has generally managed to draw that line at the right place. They have remained appropriately conservative, yet flexible – which is what you want from a medicines regulator.
Conservative here means that they have not lowered the bar for the registration of medicines, even though this would have satisfied some in the industry, and may have helped to reduce the registration backlog more quickly. At the same time, where warranted, as with the TB drug bedaquiline, they were flexible and allowed for use of the drug under controlled conditions prior to registration, since people with the worst forms of drug-resistant TB did not have any other real options.
That high regulatory standards have been maintained amidst the general doom and gloom of state capture and diminishing state capacity might well surprise some outside the health sector. We need not think twice about whether medicines in South African pharmacies are of good quality – generally they do what they claim to do, and have known side effects that you can read about in the package insert. In its most basic function of protecting the public from unsafe or unproven medicines, regulation in South Africa essentially still works.
But while this core has remained intact, the regulator has undoubtedly faced substantial challenges over the last decade or so. Most notable of these has been its slowness in registering new medicines, and the massive backlog in applications that has developed as a result.
When the Medicines Control Council (MCC) was replaced by the South African Health Products Regulatory Authority (SAHPRA) in February 2018, the backlog stood at 16,000 applications.
While speed and efficiency remain a problem, as does capacity, there are concrete signs that it is being addressed responsibly – in other words, not by lowering standards.
Last year, the regulator went on a hiring spree – something that will hopefully help increase the internal capacity of the regulator, as was envisaged with the change from the MCC to SAHPRA. They have also made explicit plans for catching up with the backlog, and reported on this to parliament. (We previously reported on their plans here.)
…the big picture seems clear enough – despite its very significant challenges, the SAHPRA has generally maintained high regulatory standards. A meaningful process of reform and capacity building is under way that should, over time, make the organisation faster, more efficient and more transparent.
Besides bolstering internal capacity, the registration process is also being speeded up by making more use of work already done by regulatory authorities in other countries where the same drugs have been registered. Using such “reliance pathways” does not negate the need for local assessments that take into account domestic laws and potential differences in how well medicines work in our population, but it can reduce much of the drudge work that goes into assessing applications.
Regarding what has arguably been the second most serious problem with our regulator in recent years – a lack of transparency – there have also been significant steps forward, though here too it remains a work in progress. More information is now routinely accessible on the SAHPRA website than was the case a year ago. Something as basic as checking whether a medicine is registered is now much easier than before. Media queries are responded to more quickly and with more openness.
In some technical areas, however, long-running problems remain.
One such issue relates to whether or not SAHPRA should take affordability into account when considering requests for section 21 authorisations. Though the MCC and SAHPRA have not been consistent on this issue, the current position seems to be that, if an unaffordably expensive drug is registered in South Africa, importing affordable generics of the same drug under section 21 authorisation should not be allowed.
A strong case could be made that the constitutional right to health requires that it should in fact be allowed under certain conditions, especially when people’s lives depend on it. As we have previously reported, this issue has directly impacted whether some cancer patients in South Africa get the medicines they need.
A second such concern relates to the difficulty in registering generics if the related originator medicine was never registered in South Africa. While this too might seem technical, the problem is currently preventing the local registration of an important new hepatitis C drug. Accordingly, people in South Africa cannot access this medication.
Both of these problems could be fixed relatively quickly, although it might require the cooperation of the Minister of Health if changes to regulations are needed.
Another important problem that is equally fixable, but that would require additional resources, is better enforcement of the law and regulations on complementary and alternative medicines. When quacks or supplement charlatans make unproven claims that their products can treat or cure HIV or Covid-19, the regulator has a duty to stop them. It is not clear whether the regulator’s lacklustre enforcement in this area is because of a lack of political will or a lack of capacity, or both.
Either way, these issues aside, the big picture seems clear enough – despite its very significant challenges, the SAHPRA has generally maintained high regulatory standards. A meaningful process of reform and capacity building is under way that should, over time, make the organisation faster, more efficient and more transparent.
Whatever disagreements one might have with specific regulatory decisions, indications are that the regulator is generally moving in the right direction. Civil society and parliament should continue to hold it accountable, but contrary to what some advocates for deregulation might argue, what accountability requires is mostly an acceleration, not a change of course. DM
Marcus Low is editor of Spotlight.
This article was produced by Spotlight – health journalism in the public interest. Sign up for our newsletter.
"Information pertaining to Covid-19, vaccines, how to control the spread of the virus and potential treatments is ever-changing. Under the South African Disaster Management Act Regulation 11(5)(c) it is prohibited to publish information through any medium with the intention to deceive people on government measures to address COVID-19. We are therefore disabling the comment section on this article in order to protect both the commenting member and ourselves from potential liability. Should you have additional information that you think we should know, please email [email protected]"





 Become an Insider
Become an Insider